Optical coherence tomography (OCT) as In-Process-Control tool – benefits during pharmaceutical manufacturing
Principles of the Technology
The essential task in the pharma part of the CAPRI project is to develop the scientific basis and implement the technologies and in particular the respective control tools, based on cognitive solutions, to steer the continuous processes in-line. In our work, as part of the pharma consortium of AMS and RCPE in the CAPRI project, we used the OCT technique.
Optical coherence tomography (OCT) is a contact-free, non-destructive, high-resolution imaging technique based on low-coherence interferometry. OCT is used to generate cross-sectional depth-resolved images of translucent materials. OCT is based on low-coherence interferometry and uses light sources with high spatial and low temporal coherence. A short coherence length is a temporal filter for back-reflected and back-scattered photons. Surface information is obtained by comparing the arrival times of single scattered photons from different sample structures with a reference light beam. [1]
OCT comes from the field of biomedicine, which is mainly used in ophthalmology, cardiology, dermatology, and gastroenterology. However, in recent years, there has been increasing interest in the use of OCT in non-destructive testing and evaluation of non-biological materials.[2] Off-line OCT applications for coating thickness determination and coating defect detection have already been studied in the literature and compared with other measurement techniques.
There are many methods available to evaluate and monitor the quality of coatings. Two groups can be distinguished among the film-coat analysis method: destructive and non-destructive. For example, scanning electron microscopy (SEM) and confocal scanning laser microscopy (CLSM) are destructive because they require a tablet to be cut and cannot be used for in-line monitoring. The second group includes several in-line spectroscopic methods such as near-infrared spectroscopy (NIRS), Raman spectroscopy, optical coherence tomography (OCT), and terahertz pulsed imaging [3] .Nondestructive techniques can provide information about the coating used, such as its thickness and uniformity, and the effect of coating properties on the dissolution rate of a drug can be investigated. Regarding the coating processes, in-process control still mainly relies on manual diameter or weight gain measurement of the coating thickness. This procedure is tedious and time-consuming. In addition, it is influenced by the operator, contains a small number of samples, and cannot take into account the variability of the tablet core. These nondestructive methods offer distinct advantages over traditional tablet weight gain calculations during coating processes to determine the amount of coating solution used.
For quick feedback, the measurement method should resolve the structure of the dosage form quickly and non-destructively. Spectroscopic techniques such as near-infrared (NIR) spectroscopy and Raman spectroscopy make it possible to characterize solid dosage forms and monitor coating processes. Combined with multivariate data analysis (MVDA), these methods enable non-invasive and quantitative real-time process monitoring.[2] The main disadvantages of spectroscopic techniques are that they do not directly provide the absolute value of the coating thickness and have a limited ability to analyze the uniformity of the coating between and within tablets. Since calibration based on primary measurements (eg. SEM) is required to provide an absolute value, the coating thickness prediction is only as accurate as reference measurements. OCT meets the requirements of an in-line measurement system, such as high sensitivity in the detection of coating layers and contamination, good transverse resolution and exceptionally high axial resolution, and high penetration depth. OCT is easy to implement, and suitable for real-time monitoring of coating quality, especially due to its high acquisition rate. The ease of use and low cost of OCT compared to other methods are attractive alternatives to in-line process monitoring. Moreover, in most cases, no sample preparation is required and therefore less waste is generated. Ultimately, process trends can be monitored without changing the base sample population.[3]
Use of OCT measurements at all stages of pre-formulation and development
Using the OCT technique, it is possible to evaluate the coating process in-line, which enables the optimization of process parameters immediately during the coating process.
In addition, after the coating is completed, it is possible to assess the uniformity of the surface and core coverage, repeatability, cracks, and pores, which is valuable information for the assessment of the tablet - it highlights imperfections that should be improved in the optimization of the tableting process as well as a possible change in the shape of the tablet (change of punches).
It is also possible to take measurements on uncoated cores and see the surface structure up close before starting the coating process.
Additionally, by comparing the images and OCT measurements with the obtained dissolution profiles, we can see correlations that can be used when planning further experiments.
After the appropriate number of batches in the experiment, certain assumptions can be made as to the expected influence of the appearance and surface characteristics of the tablets on their properties, dissolution profiles, or API bioavailability.
In the case of stability studies, by OCT imaging, it is possible to assess the state of the tablet surface after the shelf life with measurements made before the stability studies and assess how significant changes have occurred on the surface before the physical properties of tablets or dissolution profiles are performed.
The use of OCT measurements at all major stages of development and optimization of the classic film-coated tablet manufacturing process is illustrated below.
Testing of EC tablets by AMS
Picture No. 1a (7%wg)
Picture No. 1b (13%wg)
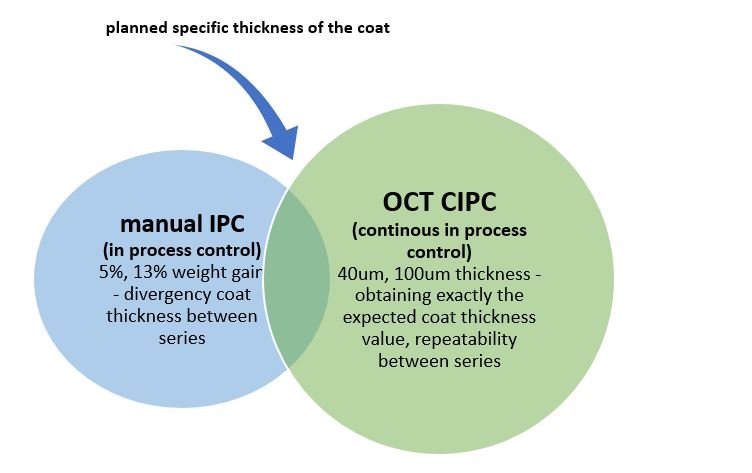
The images above show OCT results for two coat growths - samples were taken at two sampling times of the same coating process in AMS. It can be seen that it is worth working on the homogeneity of the coat by optimizing the parameters of the coating process. Pores, air bubbles, etc. appear as darker pixels in the image. Usually, the increase in the coating during the process is measured by the weight gain of a single tablet - calculated from a sufficient sample size.
Usually, the increase in the coating during the process is measured by the weight gain of a single tablet - calculated from a sufficiently large sample. Such a procedure causes many factors to escape the operator's attention. It does not obtain any information about the uniformity of the coating, the distribution of the coating growth on the edges and surface of the tablet, the pores, and the actual thickness of the layer. In-line OCT imaging is incomparably more accurate and convenient.
Two lots were manufactured based on the same formulation. The sampling method was used at four sampling times - selected based on the weight gain measurement: 10 tablets were randomly taken and weighed every 5 minutes during the coating process.
Samples were taken for four weight gains: 5%, 7%, 10%, 13%, for 150 mg cores. The process was carried out in a lab scale coater - Labcoat M. The accuracy of the test is under the influence of possibility fault because it depends to a considerable extent on the operator and the process parameters selected by him.
OCT measurements of the average thickness of the coating were obtained for tablets taken in two coating processes and compared with the average tablets' weight gains, respectively:
5% - 41,04 µm i 47,70 µm,
7% - 54,87 µm i 51,07 µm,
10% - 84,85 µm i 79,16 µm,
13% - 105,82 µm i 94,58 µm;
This shows the advantages of in-line OCT measurements, it is possible to obtain a very accurate measurement of the thickness of the coat growth and obtain high repeatability of the coating processes.
The most common defects of the tablet coating are: excessive wetting / picking - part of the coating is removed from one tablet and deposited on another, sticking two or more tablet cores together, orange peel, bridging - the film coating protrudes beyond the tablet logo, cracking, tablet abrasion / erosion, peeling, variation in thickness and heterogeneity of the coating, problems with core stability. Using the OCT technique, it is possible to successfully eliminate most tablet defects in the process, without the need to repeat the experiment.[2]
Manufacturing of pharmaceutical solid dosage forms often involves film coating as a final process. Typically, a thin continuous solid layer that controls the rate of drug release as a function of environment is applied. The main function of such modified release coatings (i.e., enteric coatings) is to adjust the initial drug release kinetics to the pH of the environment. The tablets may also be coated for visual appeal, masking an unpleasant taste, etc. A defective or too thin coating may cause the drug unusable due to ineffective gastric juice resistance. On the other hand, too much coating material may interfere with drug absorption in the small intestine. For this reason, it is so important to control the coating process as effectively as possible.[2]
A dissolution test was performed according to Ph. Eur. for enteric coated tablets.
As predicted, EC tablets survived the acid stage, and then in the buffer the dissolution profiles over time looked as in the attached graph.
For the more than twice as thick shell, "lack time" is clearly marked on the graph, and the release in the initial phase was much slower. In the OCT images, no losses in the shell or significantly uneven distribution were noticed - so the tablet behaves as expected in the medium.
In the OCT images, no losses in the coat or significantly irregular distribution were noticed - so the tablet behaves as expected in the medium.
The most common defects of the tablet coating are: excessive wetting/picking - part of the coating is removed from one tablet and deposited on another, sticking two or more tablet cores together, orange peel, bridging - the film coating protrudes beyond the tablet logo, cracking, tablet abrasion/erosion, peeling, variation in thickness and heterogeneity of the coating, problems with core stability. Using the OCT technique, it is possible to successfully eliminate most tablet defects in the process, without the need to repeat the experiment.[2]
Manufacturing of pharmaceutical solid dosage forms often involves film coating as a final process. Typically, a thin continuous solid layer that controls the rate of drug release as a function of the environment is applied. The main function of such modified release coatings (i.e., enteric coatings) is to adjust the initial drug release kinetics to the pH of the environment. The tablets may also be coated for visual appeal, masking an unpleasant taste, etc. A defective or too thin coating may cause the drug unusable due to ineffective gastric juice resistance. On the other hand, too much coating material may interfere with drug absorption in the small intestine. For this reason, it is so important to control the coating process as effectively as possible.[2]
A dissolution test was performed according to Ph. Eur. for enteric coated tablets.
As predicted, EC tablets survived the acid stage, and then in the buffer, the dissolution profiles over time looked as in the attached graph.
For the more than twice as thick shell, "lack time" is marked on the graph, and the release in the initial phase was much slower. In the OCT images, no losses in the shell or significantly uneven distribution were noticed - so the tablet behaves as expected in the medium.
In the OCT images, no losses in the coat or significantly irregular distribution were noticed - so the tablet behaves as expected in the medium.
Based on the above observations, the following conclusions can be drawn:
7% weight gain, in this case, provides a sufficiently effective enteric coat.
The 7% weight gain provides in general faster release at least during the 20 minutes. Nevertheless, release behavior of 7% and 13% don't differ significantly
Based on 1 and 2 an enteric coating weight gain of 7% is good enough to meet the critical quality attributes of an enteric coated tablet. This would have a significant cost-saving impact by reducing the material needed but also shortening the manufacturing process time needed.
Soon, with an appropriate number of experiments and a properly planned DoE, the OCT measurements will enable in general possibility to assess the quality of the functional and non-functional coating and also design a dissolution profile needed with a minimum amount of physicochemical tests.
This will significantly increase the advantage of bridging existing batch manufacturing process to a continuous manufacturing process, which is exactly the intend of the pharma consortium of RCPE and AMS in CAPRI, over standard procedure in the manufacturing process.
References:
1. Non-destructive analysis of tablet coatings with optical coherence tomography, D.M. Koller, G. Hannesschläger, M. Leitner, J.G. Khinast, European Jurnal of Pharmaceutical Sciences Volume 44, Issues 1–2, 18 September 2011, 142-148
2. Optical coherence tomography as a novel tool for in-line monitoring of a pharmaceutical film-coating proces, Daniel Markl, Günther Hannesschläger, Stephan Sacher, Michael Leitner, Johannes G. Khinast, European Jurnal of Pharmaceutical Sciences Volume 55, 13 May 2014, Pages 58-67
3. Ascertain a minimum coating thickness for acid protection of enteric
coatings by means of optical coherence tomography, European Jurnal of Pharmaceutical Sciences Volume 618, 25 April 2022, 121680